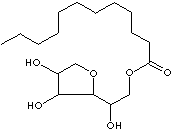
| SORBITAN
LAURATE
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRODUCT IDENTIFICATION |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| CAS NO. | 1338-39-2 |
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| EINECS NO. | 215-663-3 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FORMULA | C18H34O6 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOL WT. | 346.46 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
H.S. CODE |
3402.13 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
TOXICITY |
Oral rat LD50: 33600 mg/kg | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SYNONYMS | Sorbitan Monolaurate; Sorbitan Monoldodecanoate; | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SMILES |
Sorbitol, Fatty Acid |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
CLASSIFICATION |
SURFACTANTS / |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PHYSICAL AND CHEMICAL PROPERTIES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PHYSICAL STATE | yellow liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MELTING POINT | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| BOILING POINT |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SPECIFIC GRAVITY | 1.032 | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SOLUBILITY IN WATER | Insoluble ( soluble in mineral oil and alcohols ) | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| pH | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| VAPOR DENSITY |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HLB VALUE |
8.6 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| AUTOIGNITION |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| NFPA RATINGS |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
REFRACTIVE INDEX |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| FLASH POINT |
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| STABILITY |
Stable under ordinary conditions |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION & APPLICATIONS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| Sorbitol is a white, sweetish, hygroscopic, crystalline sugar alcohol of
six-carbon. It is found naturally in various berries and fruits. Or it is
prepared synthetically by high-pressure catalytic hydrogenation of glucose sugar
derived from cornstarch. It melts at 93 to 98 C depending on the form. It is
used as a a sweetening agent, food additive, toothpaste, tobacco, toiletries and
in cosmetics. It is used for vitamin-C fermentation. It is used as a excipient
and intravenous osmotic diuretic in pharmaceutical fields. It is also used in
the manufacture of polyethers for polyurethanes and surfactants. The term
sorbitan describes the anhydride form of sorbitol, whose fatty acids are
lipophilic whereas sorbitol body is hydrophilic. This bifunctionality in one
molecule provides the basic properties useful in cleaners, detergents, polymer
additives, and textile industry as emulsifiers, wetting agents, and viscosity
modifiers. Sorbitan esters are rather lipophilic (or hydrophobic) surfactants
exhibiting low HLB (Hydrophilic-Lipophilic Balance) values; having an affinity
for, tending to combine with, or capable of dissolving in lipids (or
water-insoluble). While, the ethoxylated sorbitan esters are hydrophilics
exhibiting high HLB values; having an affinity for water; readily absorbing or
dissolving in water. The type of fatty acid and the mole number of ethylene
oxide provides diverse HLB values for proper applications.
HLB numbers describe following characterestics: <10 : Lipid soluble (or
water-insoluble) HLB values of sorbitan compounds are:
|
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SALES SPECIFICATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
APPEARANCE |
yellow to brown liquid | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| ACID VALUE |
8.0
max
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HYDROXYL VALUE |
330
- 360
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| SAP VALUE |
150 - 175 |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| MOISTURE |
1.0%
max
|
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| TRANSPORTATION | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| PACKING |
200kgs in drum |
|||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| HAZARD CLASS | Not regulated | |||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
| UN NO. | ||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
GENERAL DESCRIPTION OF NONIONIC SURFACTANTS |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
Nonionic surfactants are surface active agents which do not dissociate into ions in aqueous solutions, unlike anionic surfactants which have a negative charge and cationic surfactants which have a positive charge in aqueous solution. Nonionic surfactants are more widely used as detergents than ionic surfactants because anionic surfactants are insoluble in many hard water and cationic surfactants are considered to be poor cleaners. In addition to detergency, nonionic surfactants show excellent solvency, low foam properties and chemical stability. It is thought that nonionic surfactants are mild on the skin even at high loadings and long-term exposure. The hydrophilic group of nonionic surfactants is a polymerized alkene oxide (water soluble polyether with 10 to 100 units length typically). They are prepared by polymerization of ethylene oxide, propylene oxide, and butylene oxide in the same molecule. Depending on the ratio and order of oxide addition, together with the number of carbon atoms which vary the chemical and physical properties, nonionic surfactant is used as a wetting agent, a detergent, or an emulsifier. Nonionic surfactants include alcohol ethoxylates, alkylphenol ethoxylates, phenol ethoxylates, amide ethoxylates, glyceride ethoxylates (soya bean oil and caster oil ethoxylates), fatty acid ethoxylates, and fatty amine ethoxylates. Another commercially significant nonionic surfactants are the alkyl glycosides in which the hydrophilic groups are sugars (polysaccharides). |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||
|
PRICES |
||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||||